The Isolation of CBD
Introduction
Many processors perform CBD isolation to create a THC-free product for their end-user for a variety of reasons. For those who have had the opportunity to isolate CBD using crystallization, it is apparent that the material will readily form crystals, sometimes almost to a fault. Controlling this crystallization becomes quite important when the user is looking to create consistent and repeatable outcomes from their process. This article will focus on a high-level background of crystallization and why specific methods are recommended for isolating CBD.
Thermodynamic Background
Crystallization can occur due to the limited solubility of materials in a solvent. Many factors come into play (including temperature, solubility, concentration, etc.), but ultimately under the right conditions, many organic compounds will begin to form organized lattices, or crystals, which are a lower energy state than when dissolved in a supersaturated solution. By controlling the various factors of the process, the crystals can form with very high concentrations of the target molecule, excluding most impurities. The ability of an organic substance to crystallize is dependent on intramolecular and intermolecular forces. For certain compounds, the result of the solidification may instead form an amorphous solid or different polymorph of the isolated product.
Types of Crystallization
There are a few ways crystallization is performed, which include precipitation, evaporative crystallization, and cooling crystallization. Precipitation can be accomplished through a reaction, pH adjustment, or using anti-solvents. These methods do not seem to be common in isolation of CBD. Evaporative crystallization is performed by removing solvent until the material hits its saturation point and begins to nucleate. While effective, evaporative crystallization is not the most ideal method for isolating CBD due to the nature of the crystallization. “When using evaporative crystallization, the process is less controlled, which can lead to CBD being isolated as a paste which can, in turn, create more issues when the process is scaled,” says Ben Schilling, Tolling Manager at Pope Scientific. Evaporative crystallization also is required to be done at vacuum conditions to ensure the CBD will form a solid instead of staying in a liquid state.
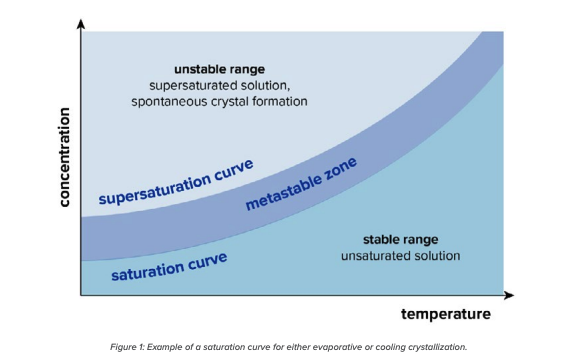
Cooling crystallization takes advantage of the fact that many organic compounds have reduced solubility with reduced temperature. Pope Scientific’s recommendation for CBD isolation is to use a jacketed reactor and temperature control unit to bring the temperature of the solution down slowly. Eventually, the solution will become supersaturated. The formation of crystals in the supersaturated solution is dependent on many factors including any impurities that may be present, the mixing profile, and whether seed crystals are utilized.
Crystallization can also be done in batches or done as a continuous process. While continuous crystallization may come out on top when considering ROI, it does add validation complications for customers operating in cGMP environments.
Considerations During Crystallization
To ensure the saturation limits of the solution are not altered significantly it is important to purify the starting material as much as possible prior to crystallization. Many users will purify their cannabinoid extract by means of wiped film molecular distillation. This process will usually allow for a distillate product to be created at > 90% cannabinoid. This purification step will increase the likelihood of consistent crystallizations.
Mixing profile in the crystallizing vessel is also very important as this can have an impact on the nucleation (formation) of crystals vs the growth stage for crystals. Depending on the ultimate use of your crystalline product, certain particle sizes and particle size distributions may be beneficial. Agitators with good pumping capabilities as well as baffles in the reactor can result in more desirable crystal formation. It is important to balance minimum solvent use with the ability to have a flowable slurry that can be mixed.
The use of seed crystals can also be vital as this can impact the quantity and size of crystals formed. Seed crystals can also be critical in preventing the formation of amorphous solids or unwanted polymorphs. Since CBD readily crystallizes, seed crystals may not be necessary for most processors.
Isolation on a Nutsche Filter Dryer
Pope Scientific recommends using a dedicated reactor for the crystallization step as this will be specifically designed for good mixing. Additionally, while the capital cost can be reduced by using a Nutsche Filter Dryer for the crystallization step, this does run the risk of clogging the filter and causing cleaning issues.
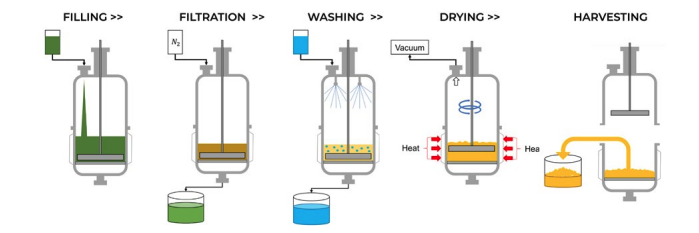
Nutsche filter dryers are valuable tools in the GMP environment for filtering, washing, and drying. After a crystal slurry is created, the Nutsche filter-dryer can be used as a single container for the multiple unit operations that are required. This is beneficial because it eliminates the risk of contamination by eliminating any transfer steps. It also reduces the amount of equipment that must be validated as clean.
First, the slurry is transferred to the Nutsche where the “mother liquor” or supernatant can be separated from the crystalline product using a fine filter screen. The advantage of a Nutsche Filter Dryer over a standard filtration device is that it can be pressurized to speed along the filtering process and mother liquor can be recirculated allowing for the complete transfer of the crystals from the reaction vessel.
After the material is filtered, a pure solvent is typically added to remove any impurities that may be present on the surface of the crystals, often called a “wash” step. Washing is performed with either a spray nozzle or ring which allows for even distribution of solvent across the filter cake. If there is not an even distribution of solvent, channeling can occur, which will reduce the effectiveness of the wash. The washed crystals at this point should be of high purity.
These crystals can then be dried by applying heat and vacuum to the Nutsche filter dryer. For many crystals, the ability to apply vacuum in the Nutsche Filter Dryer is critical as the melting point of the crystals may be lower than the boiling point of the solvent. The agitator in the Nutsche allows for the cake to be smoothed which results in even drying and through proper process development and testing can yield a reliably solvent-free product.
While the methods and ideas described above do not delve into any new territory for organic chemistry, they are important concepts to consider when developing an isolation process. Often innovation is seen as an extremely important aspect of the cannabis industry, but it is important to consider tried and true methods when there is a need to meet cGMP requirements and put out a robust and consistent product.
For more information contact Pope today!









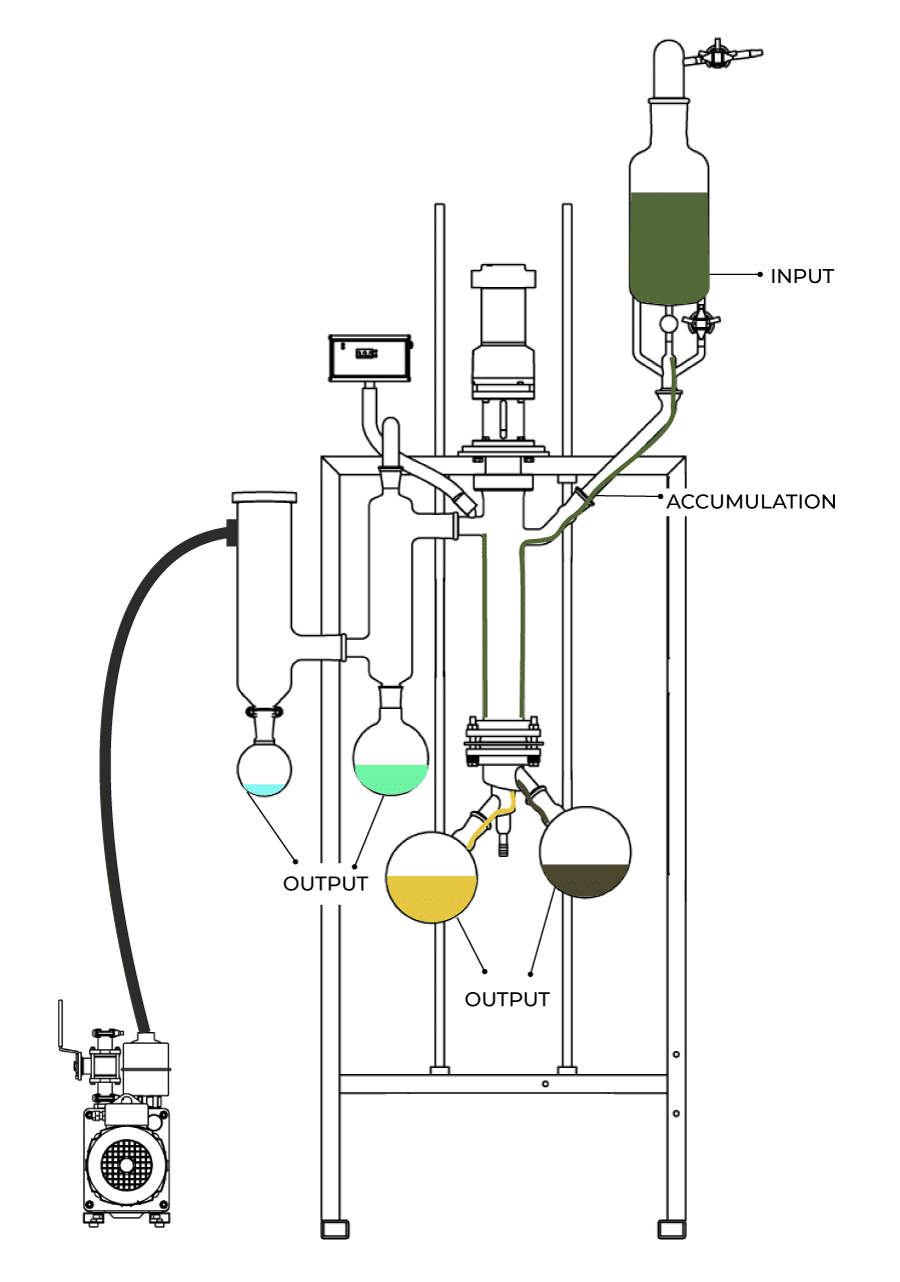

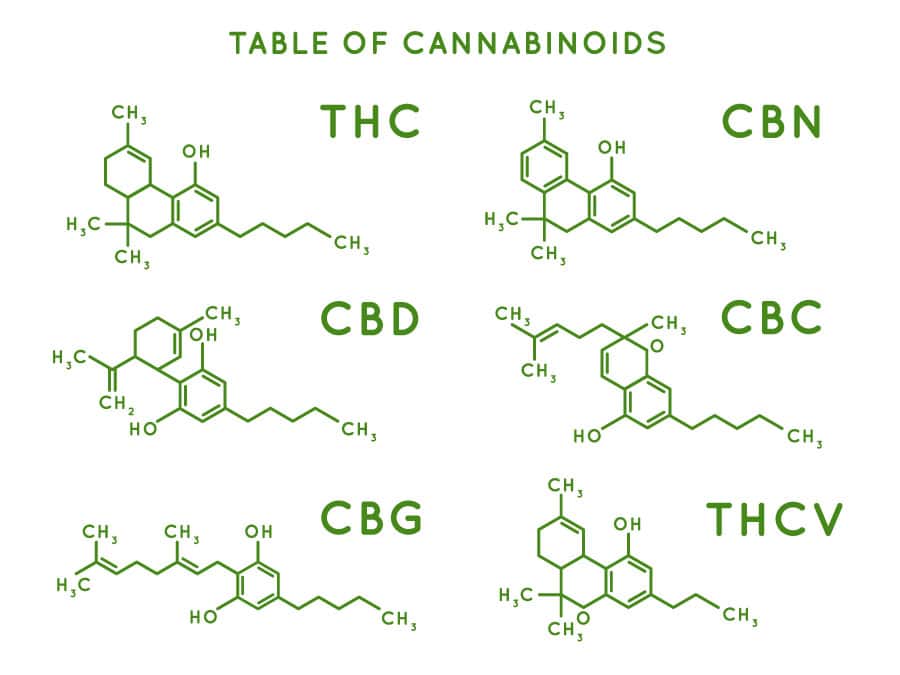


 case of post-extraction decarbing, one of the most efficient known methods is to have a batch of liquid extract contained within a stainless steel reaction vessel which is heated under vacuum conditions and rapidly stirred.
case of post-extraction decarbing, one of the most efficient known methods is to have a batch of liquid extract contained within a stainless steel reaction vessel which is heated under vacuum conditions and rapidly stirred.